Life Without Purel
Page 1 Page 2 Page 3 Page 4 Page 5 Review
Molecules are made of atoms.
Here are some models of molecules, showing how their atoms are arranged:
Water 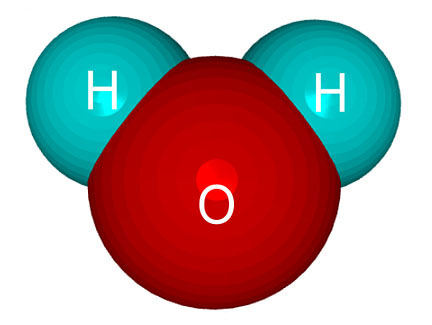 |
Fats 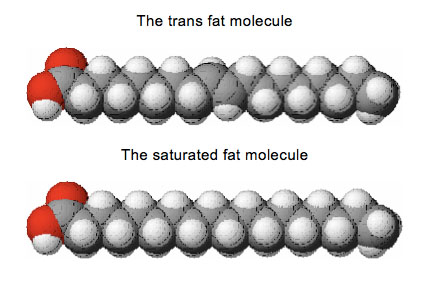 |
The mineral quartz  |
Isopropyl alcohol  |
Just as molecules are made of different atoms, atoms are made of different parts—protons, neutron and electrons. Protons have a positive (+) charge. You can think of them as similar to the north pole of a magnet.
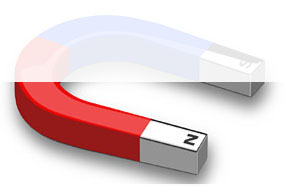
Neutrons have no charge. (We won’t worry about them for now.)
But electrons have a negative (-) charge. Think of them as similar to the south pole of a magnet.
When atoms combine to form molecules, they will often share electrons. When two hydrogen atoms combine, or "bond," with one oxygen atom to form a water molecule,
 the 2 hydrogen atoms share their electrons with the
oxygen atom. But the oxygen atom is sort of greedy, and it hugs the two
electrons from the hydrogen atoms very close to itself.
the 2 hydrogen atoms share their electrons with the
oxygen atom. But the oxygen atom is sort of greedy, and it hugs the two
electrons from the hydrogen atoms very close to itself.Since the two shared electrons are closer to the oxygen atom than the hydrogen atoms, there is more negatively-charged stuff near the oxygen part of the water molecule and less negative stuff near the hydrogen part. The result is that the molecule will have a slight negative (-) charge at one end (the oxygen end) and a slight positive charge (+) at the other end.
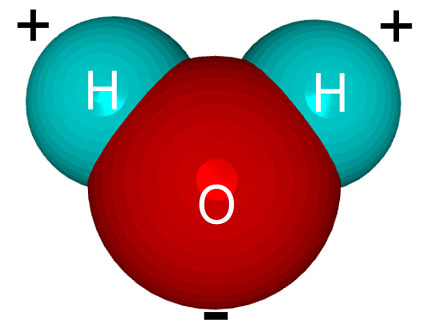
We call molecules like this “polar” molecules because the opposite ends of the molecules will attract each other just like the opposite poles of a magnet.
Now look at the fat molecules again.
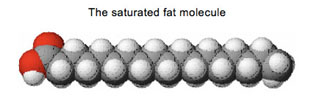 It’s a long chain of
(mostly) carbon atoms with hydrogen atoms around them on all sides.
Electrons are shared in all directions. There is no one area that has a
lot more electrons than other areas. So, unlike water molecules, there
are no magnet-like positive or negative “poles” to these molecules. We
call these kinds of molecules “non-polar.” Guess why.
It’s a long chain of
(mostly) carbon atoms with hydrogen atoms around them on all sides.
Electrons are shared in all directions. There is no one area that has a
lot more electrons than other areas. So, unlike water molecules, there
are no magnet-like positive or negative “poles” to these molecules. We
call these kinds of molecules “non-polar.” Guess why. If all this is getting too complicated, don't worry. Just remember this:
Some molecules attract each other like tiny magnets.
 These molecules are called polar molecules.
These molecules are called polar molecules.Other molecules do not attract each other. These are called non-polar molecules.
NEXT PAGE
Questions
about this page? Email: gsimonelli@leffellschool.org
Last Updated:
March 22, 2020