Life Without Purel
Page 1 Page 2 Page 3 Page 4 Page 5 Review
Getting back to Purel.
Remember: likes dissolve likes. Polar dissolves polar. Non-polar dissolves non-polar.
And that gets us back—finally—to Purel.

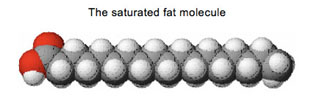
Purel, like most hand sanitizers, is made of many different chemicals, but the main chemical is isopropyl alcohol. That’s what kills the germs.
The coronavirus, as we learned in the lesson on viruses, is basically a bundle of instructions for making new viruses. Those instructions are surrounded by a layer of lipids. Here is a drawing of a typical lipid molecule:
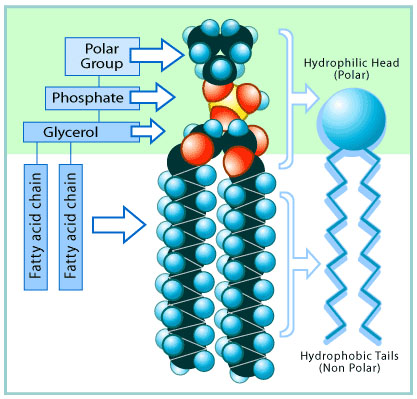
Don't let this drawing confuse you. The thing to know about lipids is that they are mostly fats.
 Most
of the molecule is non-polar, just like most other fats, but with a
polar section at one end. When the lipids surround the virus'
instructions, the lipids protect them.
Most
of the molecule is non-polar, just like most other fats, but with a
polar section at one end. When the lipids surround the virus'
instructions, the lipids protect them.This means that washing your hands with water
 alone won’t protect you from the virus very well, since the
fatty lipids (non-polar) repel the water (polar). Also, viruses are “sticky”—they cling to your skin, and plain water won’t wash them away. So you have
to destroy them.
alone won’t protect you from the virus very well, since the
fatty lipids (non-polar) repel the water (polar). Also, viruses are “sticky”—they cling to your skin, and plain water won’t wash them away. So you have
to destroy them. But plain water can’t get to the virus—that bundle of instructions—to destroy it, just like it couldn’t dissolve the oil-coated salt crystals. The non-polar fatty layer surrounding the instructions protects them from water just like the oil coating the salt crystals protected them in our experiment. That’s why many people use Purel or other hand sanitizers.
Let's look at the isopropyl alcohol molecule again:
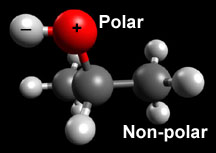
Isopropyl alcohol is mostly non-polar, but it has a section—the part with the red ball in the model—that is polar. It is the non-polar part of the alcohol molecule that allows Purel to dissolve the fatty layer that protects the virus. Once that layer is dissolved, the virus is destroyed.
The problem is that isopropyl alcohol has to be in contact with the virus for some time before the virus is destroyed. Most experts recommend that when you are trying to destroy viruses with alcohol, you should leave the alcohol on for about 30 seconds to be absolutely sure most of the viruses are gone.
Soap,
 on the other hand, (was that a pun?) has a chemical structure that
destroys the virus much faster:
on the other hand, (was that a pun?) has a chemical structure that
destroys the virus much faster: 
Notice that, like a lipid, the soap has a long non-polar section and a shorter polar section at the end.
Here is a link to an image showing how soap destroys a coronavirus. There are two terms in the illustration that we haven't talked about yet. Hydrophillic means "attracted to water." Hydrophobic means "repelled by water." In the summer when it's hot and I'm standing by a lake, I am hydrophillic. In the winter, when it's cold and raining, I am hydrophobic.
"Likes dissolve likes." A soap molecule's long, non-polar chain attacks the virus' protective fatty layer and immediately starts tearing it apart. The virus hasn’t got a chance.
This is not to say that hand sanitizers aren’t effective in killing viruses. They are. But the surprising thing is that ordinary hand soap is even better! So you won't need to spend $300 to buy that bottle of Purel from any greedy Purel hoarders!
ON TO THE REVIEW
Questions
about this page? Email: gsimonelli@leffellschool.org
Last Updated:
March 23, 2020