Life Without Purel
Page 1 Page 2 Page 3 Page 4 Page 5 Review
Let’s do an experiment:
Here is a drawing of a molecule of ordinary table salt, which is made of sodium (Na) and chlorine (Cl).
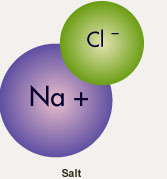
Chlorine is an electron hog, so when it bonds with sodium to make sodium chloride—regular table salt—it pulls an electron away from the sodium and holds onto it very tightly. So a typical salt molecule has a tiny positive (+) charge on one side and a tiny negative (-) charge on the other. It is a polar molecule.
And now, the experiment:
1) Pour about 3 oz. of water into a clear glass or empty glass jar.
2) Pour 3 oz. of cooking oil into a different glass or jar. (Make sure this glass can hold at least 8 oz. of liquid.)
3) Next, pour ½ teaspoon of salt into the cup with the water.
4) Stir until all the salt is dissolved. (Go ahead, I’ll wait.)
All dissolved? Good.
5) Now, add ½ teaspoon of salt to the cooking oil and stir until it is dissolved.
All dissolved yet? No?
That’s okay. Keep stirring.
Still not dissolved?
Maybe try a little more.
Okay, you can stop.
Actually, I knew that you wouldn’t be able to dissolve the salt into the cooking oil. How did I know? Chemists have an expression: “Likes dissolve likes.” By this, they mean that a liquid made of polar molecules, like water, will dissolve other things made of polar molecules, and a liquid made of non-polar molecules will dissolve stuff made of non-polar molecules. But a polar liquid cannot dissolve a non-polar substance, and vice versa.
To show this in action, pour the saltwater that you just made into the glass containing the cooking oil and salt—go ahead, try it; I’ll wait—and the oil will rise up above the water while the salt sinks to the bottom.
To see something interesting, try stirring the liquids now. See if you can dissolve the rest of the salt. You might be able to get some of the salt from the oil to dissolve in the water, but a lot of it will not dissolve. That’s because the salt crystals have gotten coated with oil. Since the oil—non-polar—repels the water—polar—the water can’t get to the salt crystals to dissolve them. The salt will just sit at the bottom of the cup.

Can you see the undissolved salt
on the bottom of this glass?
NEXT PAGE
Questions
about this page? Email: gsimonelli@leffellschool.org